structured authoring + compliance
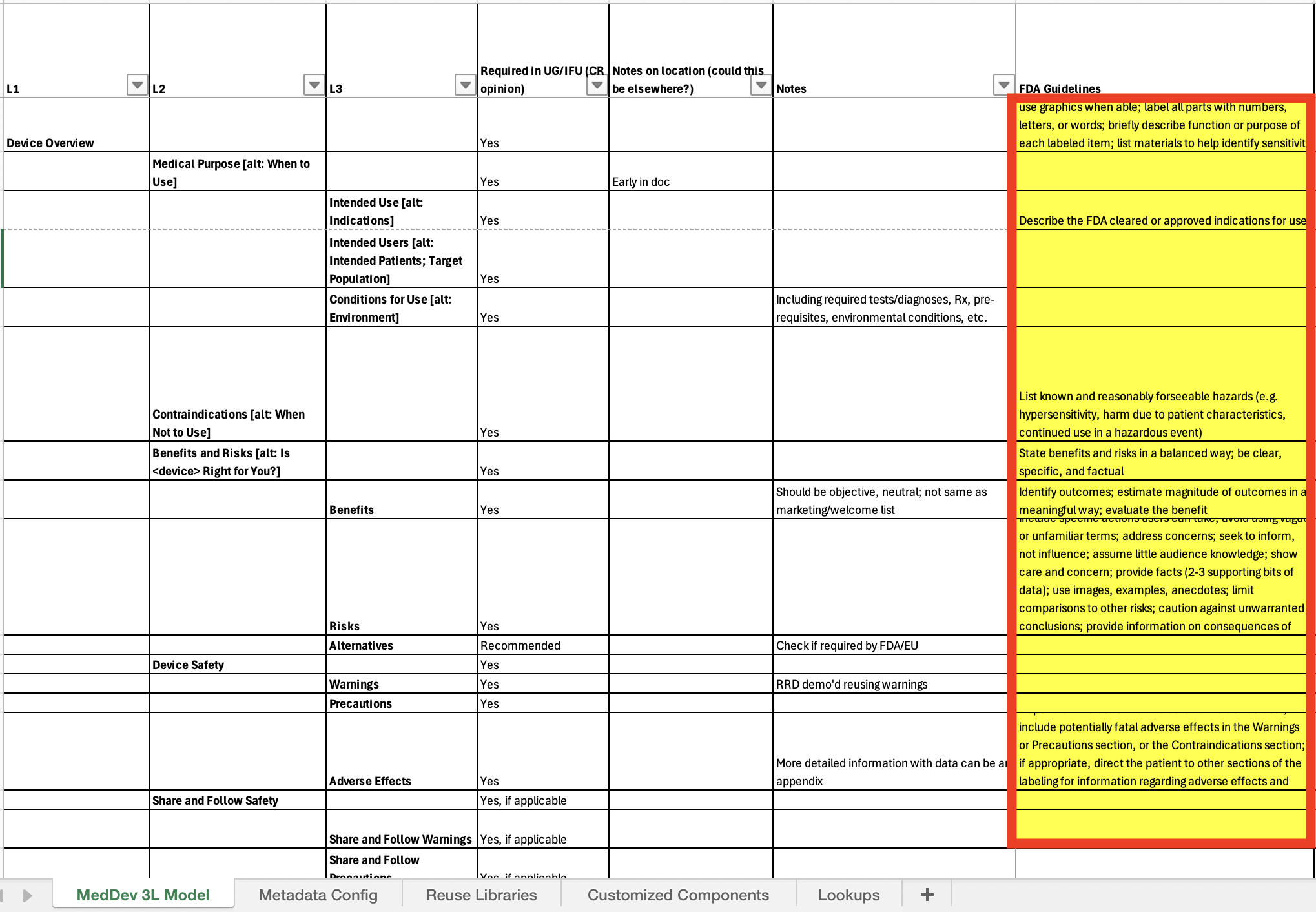
Transformed marketing material for a fictional medical device into FDA-compliant documentation, applying regulations and structured authoring to demonstrate traceability and compliance readiness.
Transformed marketing material for a fictional medical device into FDA-compliant documentation, applying regulations and structured authoring to demonstrate traceability and compliance readiness.